Lactic acid
| Lactic acid | |
|---|---|
 |
 |
|
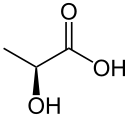
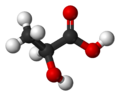
2-hydroxypropanoic acid Note: The S enantiomer is depicted in each of the structural models included above.
|
|
| Identifiers | |
| CAS number | 50-21-5, D/L: [50-21-5] L: [79-33-4] D: [10326-41-7] |
| ChemSpider | 96860 |
| ATC code | G01,QP53AG02 |
|
SMILES
CC(O)C(=O)O
|
|
| Properties | |
| Molecular formula | C3H6O3 |
| Molar mass | 90.08 g/mol |
| Melting point |
L: 53 °C |
| Boiling point |
122 °C @ 12 mmHg |
| Acidity (pKa) | 3.86 at 25 °C |
| Related compounds | |
| Other anions | lactate |
| Related carboxylic acids | acetic acid glycolic acid propionic acid 3-hydroxypropanoic acid malonic acid butyric acid hydroxybutyric acid |
| Related compounds | 1-propanol 2-propanol propionaldehyde acrolein sodium lactate |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
| Infobox references | |
Lactic acid (IUPAC systematic name: 2-hydroxypropanoic acid), also known as milk acid, is a chemical compound that plays a role in several biochemical processes. It was first isolated in 1780 by the Swedish chemist Carl Wilhelm Scheele. It is a carboxylic acid, whose chemical formula C3H6O3 has its hydroxyl group adjacent to the carboxyl group, making it an alpha hydroxy acid (AHA). In solution, it can lose a proton from the acidic group, producing the lactate ion CH3CH(OH)COO−. It is miscible with water or ethanol, and is hygroscopic.
Lactic acid is chiral and has two optical isomers. One is known as L-(+)-lactic acid or (S)-lactic acid and the other, its mirror image, is D-(−)-lactic acid or (R)-lactic acid. L-(+)-Lactic acid is the biologically important isomer.
In animals, L-lactate is constantly produced from pyruvate via the enzyme lactate dehydrogenase (LDH) in a process of fermentation during normal metabolism and exercise. It does not increase in concentration until the rate of lactate production exceeds the rate of lactate removal, which is governed by a number of factors, including monocarboxylate transporters, concentration and isoform of LDH, and oxidative capacity of tissues. The concentration of blood lactate is usually 1–2 mmol/L at rest, but can rise to over 20 mmol/L during intense exertion.
In industry, lactic acid fermentation is performed by Lactobacillus bacteria, among others. These bacteria can operate in the mouth; the acid they produce is responsible for the tooth decay known as caries.[1][2][3][4]
In medicine, lactate is one of the main components of Ringer's lactate or lactated Ringer's solution (Compound Sodium Lactate or Hartmann's Solution in the UK). This intravenous fluid consists of sodium and potassium cations, with lactate and chloride anions, in solution with distilled water in concentration so as to be isotonic compared to human blood. It is most commonly used for fluid resuscitation after blood loss due to trauma, surgery, or a burn injury.
Contents |
Exercise and lactate
During power exercises such as sprinting, when the rate of demand for energy is high, lactate is produced faster than the ability of the tissues to remove it, so lactate concentration begins to rise. This is a beneficial process, since the regeneration of NAD+ ensures that energy production is maintained and exercise can continue. The increased lactate produced can be removed in a number of ways, including:
- Oxidation to pyruvate by well-oxygenated muscle cells, which is then directly used to fuel the Krebs cycle
- Conversion to glucose via gluconeogenesis in the liver and release back into the circulation; see the Cori cycle.
Contrary to popular belief, this increased concentration of lactate does not directly cause acidosis, nor is it responsible for delayed onset muscle soreness.[5] This is because lactate itself is not capable of releasing a proton,[5] and, second, the acidic form of lactate, lactic acid, "is not produced in muscle".[6] Analysis of the glycolytic pathway in humans indicates that there is not enough hydrogen ions present in the glycolytic intermediates to produce lactic or any other acid.
The acidosis that is associated with increases in lactate concentration during heavy exercise arises from a separate reaction. When ATP is hydrolysed, a hydrogen ion is released. ATP-derived hydrogen ions are responsible primarily for the decrease in pH. During intense exercise, aerobic metabolism cannot produce ATP quickly enough to supply the demands of the muscle. As a result, anaerobic metabolism becomes the dominant energy-producing pathway, as it can form ATP at high rates. Due to the large amounts of ATP being produced and hydrolysed in a short period of time, the buffering systems of the tissues are overcome, causing pH to fall and creating a state of acidosis, a natural process that facilitates the easier dissociation of oxyhaemoglobin and allows easier transfer of oxygen from the blood.[7] This may be one factor, among many, that contributes to the acute muscular discomfort experienced shortly after intense exercise.
The effect of lactate on acidosis has been the topic of many recent conferences in the field of exercise physiology. Robergs et al. have accurately chased the proton movement that occurs during glycolysis. However, in doing so, they have suggested that [H+] is an independent variable that determines its own concentration. A recent review by Lindinger et al.[6] has been written to rebut the stoichiometric approach used by Robergs et al. (2004).[5] In using this stoichiometric process, Robergs et al. have ignored the causative factors (independent variables) of the concentration of hydrogen ions (denoted [H+]). These factors are strong ion difference [SID], PCO2, and weak acid buffers. Lactate is a strong anion, and causes a reduction in [SID], which causes an increase in [H+] to maintain electroneutrality. PCO2 also causes an increase in [H+]. During exercise, the intramuscular lactate concentration and PCO2 increase, causing an increase in [H+], and, thus, a decrease in pH. (See Le Chatelier's principle)
Polymer precursor
Two molecules of lactic acid can be dehydrated to lactide, a cyclic lactone. A variety of catalysts can polymerise lactide to either heterotactic or syndiotactic polylactide, which as biodegradable polyesters with valuable (inter alia) medical properties are currently attracting much attention.
Nowadays, lactic acid is used as a monomer for producing polylactic acid (PLA), which later has developed application as biodegradable plastic. This kind of plastic is a good option for substituting conventional plastic produced from petroleum oil because of low emission of carbon dioxide. The commonly used process in producing lactic acid is via fermentation, and, later, to obtain the polylactic acid, the polymerization process follows.
Foods
Lactic acid is found primarily in sour milk products, such as koumiss, leban, yogurt, kefir, and some cottage cheeses. The casein in fermented milk is coagulated (curdled) by lactic acid. Lactic acid is also responsible for the sour flavor of sourdough breads.
Detergents
Lactic acid has gained importance in the detergents industry the last decade. It is a good descaler, soap-scum remover, and a registered anti-bacterial agent. An economically beneficial as well as environmentally beneficial trend toward safer and natural ingredients has also contributed.
See also
- Cori cycle
- Alanine cycle
- Dental caries
- Biodegradable plastic
- 2-Hydroxybutyric acid
- Acids in wine
References
- ↑ Ecology of Lactobacilli in the Oral Cavity: A Review of Literature The Open Microbiology Journal, 2008, Vol. 2, pages 38-48.
- ↑ Correlations of oral bacterial arginine and urea catabolism with caries experience Oral Microbiol. Immunol. 2009, Vol. 24(2), pages 89–95.
- ↑ Bacteria of Dental Caries in Primary and Permanent Teeth in Children and Young Adults Journal of Clinical Microbiology, 2008, Vol. 46(4), pages 1407–1417
- ↑ Diversity of Lactobacilli in the Oral Cavities of Young Women with Dental Caries Caries Res. 2007, Vol. 41(1), pages 2–8
- ↑ 5.0 5.1 5.2 Biochemistry of exercise-induced metabolic acidosis Am J Physiol Regul Integr Comp Physiol, 2004, Vol. 287, pages R502–R516
- ↑ 6.0 6.1 Applying physicochemical principles to skeletal muscle acid-base status Am J Physiol Regul Integr Comp Physiol, 2004, Vol. 289(3), pages R890–94
- ↑ doi:10.1111/j.1399-6576.1995.tb04325.x
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand